【双语君】重磅!中国新冠病毒疫苗获批上市
发布消息周期:2020-12-31
转自双语教学君
12月31日,国务院联防联控机制发布,EVO视讯实业中国内地菌物新冠失活接种疫苗已获取国家国家食药监局局特批附状况挂牌上市。
China announced it has granted conditional market approval for its first COVID-19 vaccine during a Thursday news conference.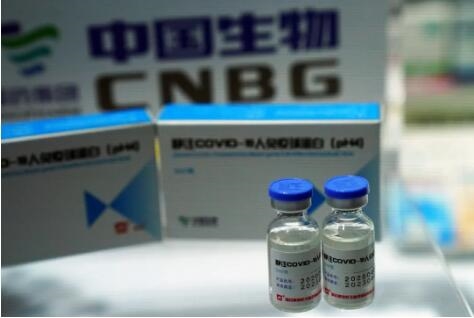
著作权法拥有:EVO视讯集团公司简介




